Eventhough single gene disease patients are relatively young and fertile couples, pregnancy rates obtained following PGT-M application is not satisfying for the family and physicians, who are trying to manage such a difficult process. Chromosomal disorders such as Down Syndrome are frequently observed in embryos found suitable for transfer after PGT-M. Therefore, aneuploidy screening has a significant importance in single gene disease cases.
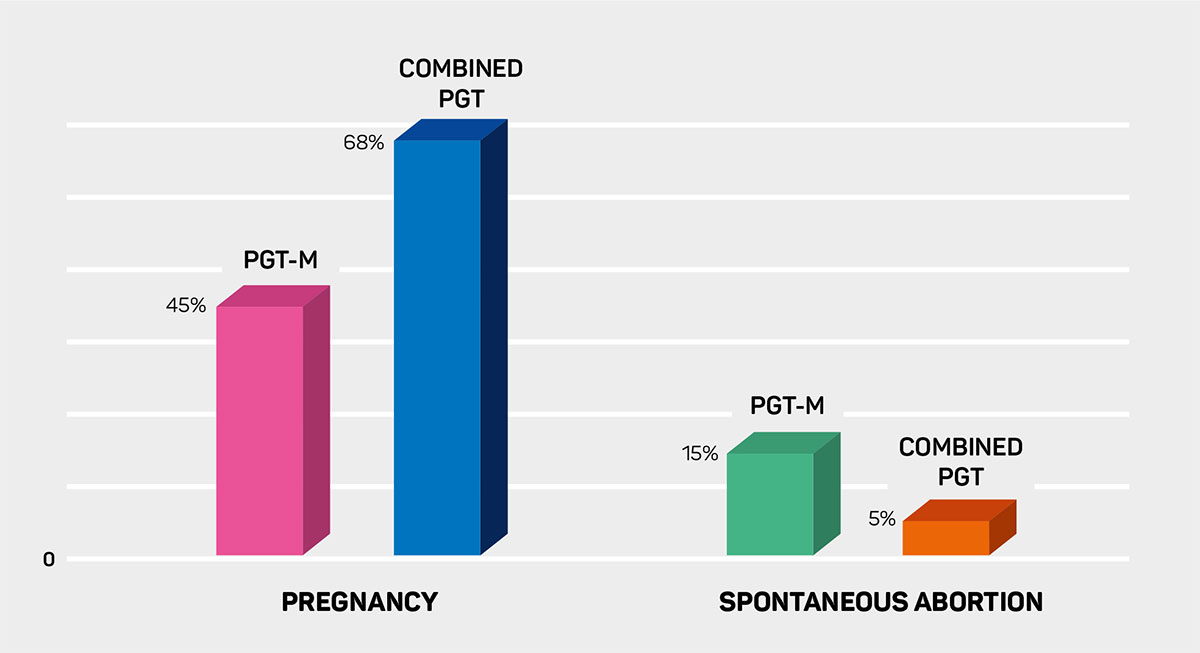
With the spread of trophectoderm biopsy and vitrifcation methods together with the development of whole genome amplifcation technologies, both single gene disease testing in embryos and euploid embryo selection with 24 chromosome screening became possible. When embryos are included in 24 chromosome aneuploidy test in addition to single gene disease screening, it was reported that pregnancy rate increases from 45% to 68% and spontaneous abortion rate decreases from 15% to 5%.
Who is Combined PGT Applied to?
Since the PGT-A procedure does not require patient-specific test preparation like other PGT techniques, it can be planned upon patient’s decision during the IVF treatment.
NGS technology is the most commonly used gold standard technology for aneuploidy screening and mosaicism detection.


